Pilot Data on the Use of Digital Behavioral Therapy for the Treatment of Type 2 Diabetes Presented at Endocrine 2020

Data from a clinical study of Better’s prescription digital therapeutic in patients with type 2 diabetes will be presented at the upcoming Endocrine 2020 Conference. The conference will take place on March 28-31, at San Francisco’s Moscone Center.
Highlights of the study include:
Mean reduction in fasting blood glucose of 21.9 mg/dL (approximately -1.0% HbA1c) over an average of 73 days between baseline and ending values.
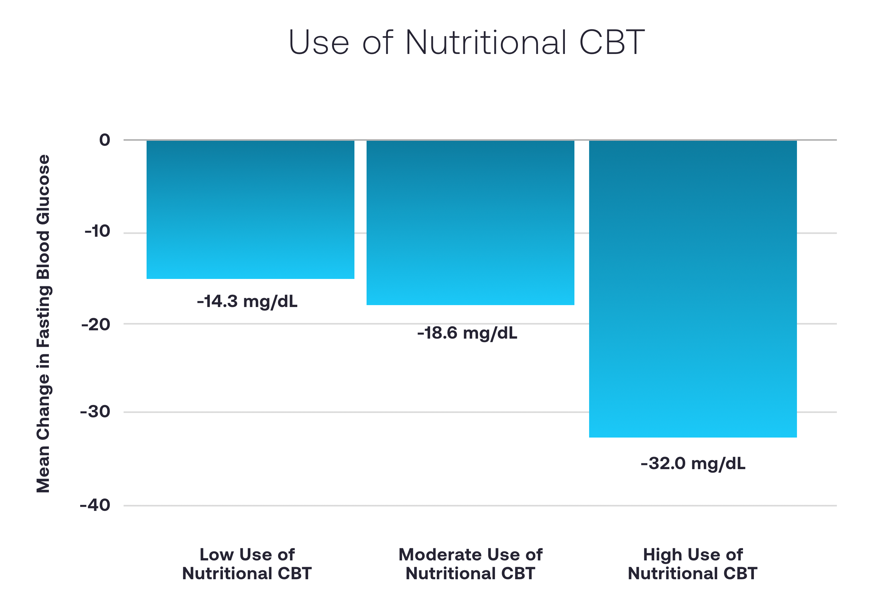
Larger improvements in fasting blood glucose are seen in participants with higher use of the behavioral therapy. An improvement of -32.0 mg/dL was observed in the highest tertile of use compared to -14.3 mg/dL in the lowest tertile.
Participants used the digital therapeutic 4.4 days a week on average, and spent an average of 6 minutes during each use. The study included 74 participants with type 2 diabetes and a baseline HbA1c ≥ 7%. Diabetes is defined by an HbA1c value of 6.5% or higher. In addition, study participants had a mean age of 55.2 years, baseline BMI of 34.7 kg/m2, and 72% were female.
“While it is commonly assumed that only newly diagnosed patients can benefit from behavioral therapy, we are encouraged to see such a strong efficacy signal in a diverse group of patients with long-standing diabetes. Study participants were recruited from 32 states and reported a mean time since diagnosis of 10.6 years,” said Dr. Mark Berman, Chief Medical Officer.
This study represents an important milestone on the path to regulatory approval, demonstrating that a software-only product configuration delivering behavioral therapy can produce meaningful outcomes in patients. This configuration productized insights captured by our health coaches and nurses into nutritional CBT modules and content. “We were optimistic but uncertain this would work, until we saw the data. Greater use of nutritional CBT resulted in greater reductions in blood sugar, and even a low level of use resulted in meaningful improvement,” said Kevin Appelbaum, CEO.
Next, Better will conduct an open-label, multi-site, randomized, controlled, parallel-group trial of this digital therapeutic for treating type 2 diabetes and submit the results as the basis of its premarket submission to the U.S. Food and Drug Administration (FDA).


